The initial stage of the nuclear fuel cycle (NFC) is the extraction of ore and the production of uranium concentrate, which includes the main stages:
the actual production of uranium-containing ore;
its mechanical enrichment by removing waste rock;
grinding the obtained ore mass;
leaching uranium from it using sulfuric acid or sodium carbonate;
obtaining uranium concentrate by extraction from uranium solutions (extraction, sorption or selective precipitation);
drying of uranium concentrate and its sealed packaging (112).
Uranium ore is mined in mines and open pits by conventional methods and by in-situ leaching, in which special solutions are introduced into the underground deposit to dissolve uranium.
All uranium mining enterprises have a negative impact on the environment. The main sources of radioactive contamination at mining sites are open pits, mines, “tailings” (a specially designated area for storing “tailings” - rock dumps after the technological process of extracting a useful component from uranium ore), open ore storages, dumps. Pollution is caused by the release of radioactive gases, dust and aerosols into the atmosphere, the discharge of mine water, leaks and accidental discharges from tailing ponds and hydrotransport systems, as well as due to the use of ore rocks as local building materials (112). In the United States, the total volume of "tailings" accounts for more than 95% of the total volume of all radioactive waste at all stages of the production of nuclear weapons and electricity. Despite the fact that the hazard from one gram of "tailings" is small compared to most other radioactive waste, the large volume of this waste and the lack of appropriate legislative measures until 1980 led to a significant increase in the level of environmental pollution (146).
Figure 26. Uranium quarry (145).
Uranium oxide (U 3 O 8) is extracted from the ore by crushing (purification), in the form of a "yellow cake" - a yellow or brown powder containing about 90% of uranium oxide.
The raw materials for obtaining nuclear fuel differ depending on the type of nuclear reactor for which the fuel is intended. Most reactors use enriched uranium, and the starting compound for its enrichment is uranium hexafluoride. Natural uranium contains 0.8% of the isotope 235U. To reduce the size of the reactor, the 235U content in the fuel is preliminarily increased to 2.0 or 2.4%.
The production of chemical concentrates of natural uranium in the form of uranium (III) octoxide U 3 O 8 or sodium diuranate Na 2 U 2 O 7 is carried out in the process of hydrometallurgical production. The choice of technology is due to the chemical composition of the ore and the specifics of the enterprise. In carbonate leaching, crushed uranium ore is processed with sodium carbonate Na 2 CO 3 to obtain a uranium solution, from which, using appropriate chemical reactions, the selective precipitation of uranium in the form of sodium diuranate is carried out. After additional purification of the product, it is dried, and the resulting yellow powder is packed in sealed containers (112).
Another type of uranium concentrate - uranium (III) octoxide U 3 O 8, after drying, is a black powder and is also packed in sealed containers.
The uranium concentrate obtained at the first stage of the nuclear fuel cycle goes to chemical processing, where the batches of concentrate are averaged and cleaned of impurities. Prior to the implementation of the isotopic enrichment process, it is necessary to carry out an operation of additional purification of uranium to convert it into a nuclear-grade material (such an operation is called refining). Particular attention is paid to the purification of uranium from boron, cadmium, hafnium, which are neutron-absorbing elements, as well as from rare earth elements (gadolinium, europium and samarium). Technologically, refining consists in the extraction purification of uranium with tributyl phosphate after dissolving the uranium concentrate in nitric acid (143).
The end product of chemical processing is uranium tetrafluoride, which is sent for conversion. At present, uranium hexafluoride, in terms of its combination of properties, is the most suitable chemical compound for isotopic enrichment using the developed technologies. It includes the production of pure fluorine, grinding tetrafluoride (UF4) or uranium oxide to a powder state, followed by its combustion in a fluorine torch. Then, uranium hexafluoride (UF 6) is filtered and condensed in a system of cold traps. Uranium hexafluoride is enriched in the uranium-235 isotope.
Uranium enrichment enterprises are part of the TVEL Fuel Company, which unites all enterprises and organizations, one way or another related to the production of nuclear fuel (45).
Four enterprises are directly involved in uranium enrichment:
Angarsk Electrolysis Chemical Plant (Angarsk, Irkutsk Region)
Production Association "Electrochemical Plant"
(Zelenogorsk, Krasnoyarsk Territory)
Ural Electrochemical Plant (Novouralsk, Sverdlovsk Region)
Siberian Chemical Combine (Seversk, Tomsk Region).
Their production capacities allow Russia, represented by Rosatom, to occupy 40% of the world market for uranium enrichment services and plan to increase this share.
Russia has the most advanced uranium enrichment technology - gas centrifuge. Inside the spinning centrifuge, heavier molecules containing U-238 atoms tend to move towards the outside of the cylinder, while lighter molecules containing U-235 remain closer to the central axis. The gas in this cylinder then begins to circulate from bottom to top, pushing depleted uranium, which is closer to the outer wall, towards the top, and gas enriched in U-235 from the center towards the bottom. Then two streams, one rich and the other lean, can be recovered from the centrifuge and separated into gaseous diffusion "cascades" (144).
Uranium dioxide powder is produced from enriched uranium hexafluoride. The U-235 enriched UF 6 arrives at the plant in 2.5 ton steel containers. UO 2 F 2 is obtained from it by hydrolysis, which is then treated with ammonium hydroxide. The precipitated ammonium diuranate is filtered off and fired to obtain uranium dioxide UO 2, which is pressed and sintered into small ceramic tablets. The range of tablets (depending on the size and enrichment) is more than 40 varieties. They are completed in batches and checked for compliance with technical requirements.
The tablets are placed in tubes made of zirconium alloy (zircaloy) and fuel rods are obtained - fuel elements (fuel rods) (Fig. 27), which are combined in about 200 pieces into complete fuel assemblies ready for use at nuclear power plants.

Drawing 27. General view of certain types of TVELs (147).
Similar technologies are used for the production of uranium-erbium pellets for fuel assemblies for RBMK reactors, as well as for the manufacture of uranium-gadolinium pellets for fuel assemblies with a burnable reactor absorber. Uranium-gadolinium fuel made it possible to increase the safety of operation of nuclear reactors and increase their fuel cycle (up to 4 years for VVER-1000 and up to 5 years for VVER-440).

Figure 28. Fuel assembly (148).
Fuel for a VVER-type reactor is a bundle of fuel elements with cladding made of zirconium alloy and enclosed in them uranium dioxide pellets. The fuel assembly (FA) for VVER reactors has a hexagonal section (Fig. 28). In addition to the fuel rods, its elements are the head, the shank, the spacer grids and, in some cases, the cover.
The head is designed for adhesion during loading and unloading, and the shank ensures the installation of fuel assemblies in the reactor and organizes a path for supplying a coolant that cools the fuel elements. VVER-440 fuel assemblies consist of 126 fuel rods. The fuel assembly for the VVER-1000 reactor includes 311-312 fuel elements. There are various fuel modifications for this type of reactors, designed for three-, four- and five-year fuel cycles.
One of the ways to improve the operational characteristics of a VVER reactor is to switch to cermet fuel, i.e. creation of a cladding fuel element based on cermet fuel with a matrix structure.
Cermet fuel is uranium dioxide granules (volume fraction of UO2 up to 70%), located in a metal matrix, usually made of a zirconium-based alloy. Such a fuel is characterized by the absence of direct contacts between the fuel particles due to their uniform distribution in the metal matrix. This is achieved by using spherical fuel particles pre-coated with a matrix material, which are pressed into cores (143).
In addition to the above scheme for the production of uranium fuel - from the mine through insignificant enrichment to fuel elements - in the last decade, reactor fuel has been made from highly enriched weapons-grade plutonium by diluting it.
Russia inherited from the USSR 25-30 thousand tactical and strategic nuclear warheads. In accordance with international agreements on the reduction of strategic and tactical nuclear weapons, the country must dismantle 16-18 thousand nuclear warheads. After dismantling the warheads, hundreds of tons of highly enriched uranium (HEU) and tens of tons of plutonium are released. At the beginning of the 21st century, the HEU reserves in Russia were estimated at 900 tons.
Dismantling of nuclear weapons is carried out at the same factories where they were created. As a result of dismantling, a nuclear material pellet is extracted from the warhead, the so-called "pit" (metallic uranium in a shell of refractory metal). In Tomsk-7, uranium metal is converted into shavings, which are sent to the Ural Electrochemical Plant. There, metallic highly enriched uranium is converted into UF 6. At the mixing unit, 235 UF 6 flows through the first pipe. The dilution is carried out not with natural uranium-238, but with poorly enriched uranium (UF 6 with 1.5% uranium-235 enrichment goes through the second pipe). As a result, at the outlet of the third pipe there is UF 6 enriched to 4 - 5% - a typical enrichment for fuel of reactors of nuclear power plants. Then, according to the usual scheme, hexafluoride is converted into uranium dioxide (144).
To dilute one kilogram of highly enriched uranium, you need about 300 kg of natural uranium. From one kilogram of highly enriched uranium, approximately 30 kg of low enriched uranium is obtained. Over 6 years, 125 tons of Russian highly enriched uranium has been diluted, which is equivalent to about 5,000 warheads. Since 1999, they began to process 30 tons per year. It is planned to process 500 tons of uranium recovered from Russian weapons within 20 years.
Currently, due to the depletion of uranium-235 reserves (both ore and storage), more and more attention is attracted by plutonium-239, as the basis for future reactor fuel, since one gram of plutonium is equivalent to 100 grams of uranium extracted from spent nuclear fuel, 1500-3000 cubic meters of natural gas, 2-4 tons of coal or one ton of oil. At the same time, plutonium is a dangerous radioactive material that can be used to create nuclear charges. Therefore, its accumulation is not only wasteful, but also dangerous. The plutonium management problem is part of the overall nuclear disarmament process, during which significant quantities of weapons-grade fissile materials - highly enriched uranium and plutonium - are released in Russia and the United States.
Plutonium dioxide, a mixture of plutonium carbides with uranium carbides, and plutonium-metal alloys are usually used to prepare nuclear fuel. However, it is more often used in the form of a mixture with natural uranium or with uranium slightly enriched in 235U (the so-called mixed oxide fuel or MOX fuel).
Mixed oxides (MOX) is a reactor fuel consisting of a mixture of uranium and plutonium oxides. MOX is used to regenerate reprocessed spent fuel (after waste separation) in slow nuclear reactors (thermal regeneration) and as fuel for fast breeder reactors (144).
Plutonium suitable for use in power reactors can be obtained by reprocessing spent nuclear fuel or from nuclear weapons.
The total amount of plutonium stored in the world at the beginning of the 21st century in all possible forms is estimated at 1239 tons, of which two-thirds is in spent nuclear fuel from nuclear power plants. Already, more than 120 thousand tons of spent nuclear fuel is stored in storage, and by 2020 it will be 450 thousand tons.
The most acceptable chemical form of plutonium when used as fuel for power reactors is plutonium dioxide PuO 2 mixed with natural uranium dioxide UO 2.
Mixed oxide fuel is commonly used in two types of reactors - fast breeder reactors (BN) and light water reactors (LWR). Typically MOX containing 5 to 8% plutonium is used in pressurized water reactors and boiling water reactors.
MOX tablets can be produced by mechanical mixing of the initial powders of uranium and plutonium dioxides with the formation of a "main mixture" of UO 2 -PuO 2. The plutonium content of the mixture is then adjusted for use in the reactor by the addition of UO 2. This technology provides a homogeneous tablet structure with increased density. The powder is then compressed and sintered to form granules, which are pressed into fuel rods (143, 144).
It is possible to reprocess weapons-grade plutonium by methods of "water" chemistry, which are well developed at plants producing plutonium - dissolving metallic plutonium in acids (a mixture of HNO 3 + HF or a mixture of HNO 3 + HCOOH or HCl), followed by purification of plutonium in the form of a nitric acid solution. From purified nitrate, PuO2 can be obtained through oxalate precipitation, or mixed oxide (U, Pu) O 2 by co-precipitation of uranate and ammonium plutonate in the presence of surfactants, or plasma denitration. This technology produces low-dusting granules. When pressing tablets, a dry binder is used - zinc stearate, which significantly improves the technological process and improves the quality of the tablets. Water methods are distinguished by the multistage and duration of the technological cycle, as well as the bulkiness of the equipment. The high aggressiveness of the solutions imposes severe restrictions on construction materials. The main problem of water technologies was and remains the formation during processing of huge amounts of highly toxic long-lived radioactive waste.
More advanced methods of reprocessing weapons-grade metallic plutonium into compounds suitable for the manufacture of fuel components for fast reactors are "non-aqueous" - pyrochemical and pyroelectrochemical technologies.
Pyrochemical method - hydrogenation of metallic plutonium followed by oxidation to PuO 2 in one reactor; pyroelectrochemical - dissolution of metallic plutonium in a molten chloride (NaCl + KCl), followed by precipitation crystallization of PuO 2 in one electrolyzer.
The essence of the technology is to reduce the number of operations and the level of environmental impact. This is achieved by introducing metallic plutonium into a molten salt medium, where it is dissolved and a finished composition for equipping fuel elements is obtained. The minimization of the impact on the environment occurs in two directions: in the molten salt, its components interact with the formation of complexes. This reduces the level of aerosol formation by 1000 times; crystalline oxides used in the production of MOX fuel regenerate aerosols 15,000 times less than powders obtained by the wet scheme. This means that protection barriers are cheaper and more reliable (156).
With high production efficiency, they have a minimal adverse effect on the environment. In the process of pyrochemical processing of plutonium, thousands of times less radioactive waste is generated in comparison with water technologies. In addition, pyrochemical technologies are more transparent from the point of view of control over the irreversibility of dismantling excess nuclear charges and control over the non-proliferation of nuclear weapons.
Occupational safety and health issues when working with MOX are more significant than in the case of uranium fuel. Plutonium isotopes differ significantly in their nuclear properties from uranium isotopes. These differences lead to the following safety implications for the MOX reactor (156):
Increased criticality - The risk of criticality in the handling and production of plutonium is much higher than in the case of uranium.
Reducing the absorption capacity of control rods (these rods absorb excess neutrons, preventing the transition to an uncontrolled chain reaction) of light water reactors. This is due to the fact that MOX absorbs low-energy neutrons (slow neutrons) relatively well, so the average neutron energy is higher, and the control rods absorb fast neutrons worse than slow neutrons. For the same reason, the absorption capacity of boron added to the coolant of a pressurized water reactor (as well as, in emergency situations, a boiling water reactor decreases. basically, it is because of this that more than one third of the uranium fuel loaded into the reactor cannot be replaced with MOX.) When using MOX, the thermal reactor is less stable, it is more difficult to stop it. type VVER.
Strengthening the negativity of some reactivity coefficients at a low degree of plutonium enrichment: the reactivity coefficient describes the change in the fission reaction rates (and, therefore, power) as a result of various changes in the situation in the core, such as the appearance of voids in the coolant, changes in the temperature of the moderator (water), temperature fuel, etc.
Strengthening the peak power. Due to the intense absorption of slow neutrons by plutonium, there is a tendency for an uneven power distribution in the core, with a maximum at the interface between UO 2 and MOX, and especially at the interface between water and MOX fuel. To mitigate this effect, special core configurations are used with specially selected gradually varying enrichment levels within the fuel assembly. This greatly complicates the manufacture of fuel rods and their integration into an assembly; if a mistake is made, there is a danger of an accident.
Reducing the fraction of delayed neutrons. Some of the neutrons are emitted immediately during the decay of the nucleus (they then exist, on average, one more microsecond), and some are emitted from the nuclei resulting from nuclear fission, with a delay from tenths of a second to tens of seconds. Although the fraction of delayed neutrons is small (0.7% or less), control over the course of the chain reaction by moving the control rods, which cannot move very quickly, is possible only due to these delayed neutrons. For 239Pu, the fraction of delayed neutrons is approximately three times less than for 235U, which complicates the control task (especially at high concentrations of 239Pu).
Acceleration of wear of reactor materials. Since, as indicated above, the use of MOX leads to an increase in the average neutron energy, which in turn “accelerates the processes of radiation destruction of reactor materials by neutrons. As a result, the service life of the reactor parts is reduced, which, under certain conditions, can create an accident hazard. "
When using MOX, the amount of plutonium in the core increases, and the radiological consequences are more dangerous. Suffice it to mention that the radiation hazard from fresh MOX fuel is much higher than that from fresh uranium fuel. Likewise, spent MOX fuel is much more dangerous than spent uranium fuel (due to the increased content of plutonium and other transuranium elements)
Higher levels of heat release and neutron radiation increase the complexity of the transportation, storage, and use of MOX fuel.
The technologies associated with the final disposal of this material have not been developed, there is only the option of immobilizing plutonium (mixing with high-level waste and liquid glass / ceramics). The final disposal of plutonium is challenging due to higher heat release, neutron radiation and criticality. Due to the high content of plutonium and other transuranic elements, the disposal of MOX is much more difficult, more dangerous and more expensive than the disposal of traditional spent nuclear fuel (156).
Is it true, you say, that no one needs natural uranium? Let's take a look at consumption.
At the moment, the following types of enriched uranium are in demand in the world:
- 1. Natural uranium (0.712%). Heavy water reactors (PHWR) such as CANDU
- 2. Weakly enriched uranium (2-3%, 4-5%). Reactors of the water-graphite-zirconium, water-water-zirconium type, VVER, PWR, RBMK reactors
- 3. Medium enriched uranium (15-25%), fast reactors, transport reactors (icebreakers, floating nuclear power plants)
- 4. Highly enriched uranium (> 50%), three nuclear power plants (submarines), research reactors.
Natural uranium only goes through the first point. If we assume that in our world, uranium consumers are only commercial reactors, then PHWR of them is less than 10%. And if you count everything else (transport, research), then ... in short, natural uranium is neither to the village nor to the city. This means that almost any consumer requires an increase in the percentage of the light isotope in the 235-238 mixture. Moreover, uranium is used not only in nuclear power, but also in the production of armor, ammunition, and something else. And there it is better to have depleted uranium, which in principle requires the same processes, just the opposite.
There will be an article about the methods of enrichment.
As a raw material for enrichment, not pure uranium metal is used, but uranium hexafluoride UF 6, which, by its combination of properties, is the most suitable chemical compound for isotopic enrichment. For chemists, we note that uranium fluorination occurs in a vertical plasma reactor.
Despite all the abundance of enrichment methods, today only two of them are used on an industrial scale - gas diffusion and centrifuges. In both cases, gas is used - UF 6.
Closer to the case of isotope separation. For any method, the efficiency of isotope separation is characterized by the separation coefficient α - the ratio of the fraction of the “light” isotope in the “product” to its fraction in the primary mixture.
For most methods, α is only slightly larger than unity; therefore, to obtain a high isotopic concentration, a single isotope separation operation has to be repeated many times (cascades). For example, for the gas diffusion method α = 1.00429, for centrifuges the value strongly depends on the peripheral speed - at 250m / s α = 1.026, at 600m / s α = 1.233. Only with electromagnetic separation α is 10-1000 per 1 separation cycle. A comparative table for several parameters will be at the end.
The entire cascade of enrichment machines is always divided into stages. In the first stage of the separation cascade, the feed stream is split into two streams: lean (removed from the cascade) and enriched. Enriched is fed to the 2nd stage. At the 2nd stage, the once enriched stream is subjected to separation for the second time:
the enriched flow of the 2nd stage enters the 3rd, and its depleted flow returns to the previous (1st), etc. From the last stage of the cascade, a finished product with the required concentration of a given isotope is taken.
I will briefly tell you about the main separation methods that have ever been used in the world.
Electromagnetic separation
Using this method, it is possible to separate the components of a mixture in a magnetic field, and with high purity. Electromagnetic separation is historically the first method mastered for the separation of uranium isotopes.
Since the separation can be performed with uranium ions, the conversion of uranium to UF 6, in principle, is not necessary. This method gives high purity but low yield at high energy costs. The substance, the isotopes of which you want to separate, is placed in the crucible of the ion source, evaporated and ionized. Ions are pulled out of the ionization chamber by a strong electric field. The ion beam enters the vacuum separation chamber in a magnetic field H directed perpendicular to the movement of the ions. As a result, the ions move in their circles with different (depending on the mass) radii of curvature. It is enough to look at the picture and remember the school lessons, where we all counted along what radius an electron or a proton would fly in a magnetic field.
Diagram showing the principle of electromagnetic separation.
The advantage of the method is the use of a relatively simple technology (calutrons: CAL ifornia U niversity).
It was used for uranium enrichment at the Y-12 plant (USA), had 5184 separation chambers - "calutrons", and for the first time made it possible to obtain kilogram quantities of 235U of high enrichment - 80% or higher.
In the Manhattan Project, calutons were used after thermal diffusion - the alpha calutrons received 7% raw material (Y-12 plant) and were enriched to 15%. Weapon-grade uranium (up to 90%) was obtained from beta calutrons at the Y-12 plant. Alpha and beta calutrons have nothing to do with alpha and beta particles, they are just two "lines" of calutrons, one for preliminary, the second for final enrichment.
The method allows you to separate any combination of isotopes, has a very high degree of separation. Two passes are sufficient for enrichment above 80% from lean matter with an initial content of less than 1%. The productivity is determined by the value of the ion current and the efficiency of trapping ions - up to several grams of isotopes per day (in total for all isotopes).

One of the workshops of electromagnetic separation in Oak Ridge (USA)

Giant alpha calutron of the same plant
Diffusion methods
Diffusion methods were used for initial enrichment. Along with the electromagnetic method, it is historically one of the first. The diffusion method is usually understood as gas diffusion - when uranium hexafluoride is heated to a certain temperature and passed through a "sieve" - a special filter with holes of a certain size.
If a gas consisting of two types of molecules (in our case, two isotopes) is passed through a small hole or through a grid consisting of a large number of small holes, it turns out that the lighter gas molecules pass in a greater amount than the heavy ones. It is essential to note that this phenomenon occurs only when molecules pass through the hole without colliding in it, ... that is, when the free path of the molecule is greater than the diameter of the hole. Accordingly, the gas passing by the grids turns out to be depleted in light molecules. In practice, the reverse percolation of gas through the grid always takes place, as a result of which, in reality, the increase in the concentration of the light isotope (enrichment) turns out to be somewhat smaller.
The key point here is the phrase about the size of the holes. Initially, the meshes were made mechanically, as now - no one knows. Moreover, the material should work at an elevated temperature, and the holes themselves should not be clogged, their size should not change under the influence of corrosion, etc. The technologies for manufacturing diffusion barriers are still classified - the same know-how as with centrifuges.
More details under the spoiler, from the same report.
"On the state of research and practical work of Laboratory No. 2 for the production of uranium-235 by the diffusion method"
The enrichment is the greater, the greater the pressure drop across the mesh. The pressure drop is usually created by a compressor (pump) that moves the gas between the grids. Such a system, consisting of grids and a compressor driving the gas, is the separation stage.
We use uranium hexafluoride as a gas. It is a salt with a fairly high vapor pressure at room temperature. As for the nets, the requirement is imposed on them that their hole diameter be less than the mean free path of uranium hexafluoride molecules. The latter, as is well known, is inversely proportional to the gas pressure. At atmospheric pressure, the free path of molecules is approximately 1/10000 mm. Therefore, if we could make a fine mesh with holes less than 1 / 10,000 mm, we could work with gas at atmospheric pressure.
Nowadays we have learned how to make meshes with openings of about 5/1000 mm, i.e. 50 times the mean free path of molecules at atmospheric pressure. Consequently, the gas pressure at which the separation of isotopes on such grids will occur should be less than 1/50 of the atmospheric pressure. In practice, we propose to work at a pressure of about 0.01 atmospheres, i.e. in a good vacuum. Multiple gas enrichment during continuous operation can be carried out using a cascade installation consisting of a large number of stages connected in series. Calculation shows that to obtain a product enriched to a concentration of 90% with a light isotope (this concentration is sufficient to obtain an explosive), it is necessary to combine about 2000 such stages in a cascade. In a machine designed and partially manufactured by us, it is expected to receive 75-100 g of uranium-235 per day. The installation will consist of approximately 80-100 “columns”, each of which will contain 20-25 steps. The total area of the grids (the area of the grids is determined by the performance of the entire installation) will be about 8000 m 2. The total power consumed by the compressors will be 20,000 kW.
In addition, a good vacuum, which requires a sufficiently large capacity of the compressor equipment, and the presence of a large number of equipment for monitoring the tightness (which, in principle, is not a problem in the modern world, but the article was about the post-war period where everything was needed, immediately and quickly).
It was used as one of the first stages of enrichment. In the Manhattan project, the K-25 plant enriched uranium from 0.86% to 7%, then the raw materials went to calutrons. In the USSR - the long-suffering D-1 plant, as well as the D-2 and D-3 plants that followed, and so on.
Also, under the "diffusion" separation method is sometimes understood liquid diffusion - also, only in the liquid phase. The physical principle is that lighter molecules gather in a warmer area. Typically, a separator column consists of two coaxial tubes that are maintained at different temperatures. The mixture to be separated is introduced between them. The temperature difference ΔТ leads to the appearance of convective vertical flows, and a diffusion flux of isotopes is created between the surfaces of the pipes, which leads to the appearance of a difference in the concentration of isotopes in the cross section of the column. As a result, lighter isotopes accumulate at the hot surface of the inner tube and move upward. Thermal diffusion method makes it possible to separate isotopes in both gaseous and liquid phases.
In the Manhattan project, this is the S-50 plant - it enriched natural uranium to 0.86%, i.e. only 1.2 times increased the enrichment of the fifth uranium. In the USSR, work on liquid diffusion was carried out by the Radium Institute in the post-war period, but this direction did not receive any development.

A cascade of machines for gas diffusion isotope separation.
Signatures on the patent - F. Simon, K. Fuchs, R. Peierls.
Aerodynamic separation
Aerodynamic separation is a kind of centrifugation, but instead of swirling the gas, it swirls in a special nozzle. Instead of a thousand words - see the picture, the so-called. "Becker nozzle" for aerodynamic separation of uranium isotopes (a mixture of hydrogen and uranium hexafluoride) at reduced pressure. Uranium hexafluoride is a very heavy gas and leads to wear of small parts of the nozzles (see scale), and can become solid in areas of increased pressure (for example, at the inlet to the nozzle), therefore the hexafluoride is diluted with hydrogen. It is clear that at a 4% content of raw materials in the gas, and even at reduced pressure, the effectiveness of this method is not great. This method was developed and tried in South Africa and Germany.

Everything you need to know about aerodynamic separation is in this picture.

Nozzle options
Gas centrifugation
Probably every person (and a geek, even more so!) Who has heard at least once nuclear energy, bombs and enrichment, knows in general terms what a centrifuge is, how it works, and that there are many difficulties, secrets and know-how in the design of such devices. Therefore, I will say just a few words about gas centrifugation. However, to be honest, gas centrifuges have a very rich history of development and deserve a separate article.
The principle of operation is separation by centrifugal forces depending on the absolute difference in mass. During rotation (up to 1000 r / s, circumferential speed - 100 - 600 m / s), heavier molecules go to the periphery, lighter ones - in the center (near the rotor). This method is currently the most productive and cheapest (based on the price of $ / EPP).
Google is replete with schematic pictures of the centrifuge device, I will just give a couple of photos of how the assembled cascade looks like. In such a room, by the way, it is quite hot - uranium hexafluoride is far from room temperature there, and the entire cascade must also be cooled.

Cascade of URENCO centrifuges. Large, under 3 meters in height.

There are also smaller ones, about half a meter. Our domestic.

To understand the scale, or what a "workshop from horizon to horizon" is.
Laser enrichment
The physical principle of laser enrichment is that the atomic energy levels of different isotopes differ slightly.
This effect can be used to separate U-235 from U-238, both in the atomic form - AVLIS, and in the molecular form - MLIS.
The method uses uranium vapors, and lasers that are precisely tuned to a specific wavelength, exciting the atoms of the 235th uranium. Then the ionized atoms are removed from the mixture by an electric or magnetic field.
The technology is very simple, and, generally speaking, does not require any super-complex mechanical devices such as diffusion grids or centrifuges, there is one problem and another.
In September 2012, Global Laser Enrichment LLC (GLE) - a consortium of General Electric, Hitachi and Cameco - received a license from the US Nuclear Regulatory Commission (NRC) to build a laser separation plant with a capacity of up to 6 million SWU at the site of an existing joint venture between GE, Toshiba and Hitachi Fuel Fabrication Plant in Wilmington, North Carolina. Planned enrichment - up to 8%. However, licensing was suspended due to problems with the spread of technology. Modern enrichment technologies (diffusion and centrifugation) require special equipment, so special that, generally speaking, if desired, through the monitoring of international contracts, one can indirectly assume who is going to "quietly" (without the IAEA's knowledge) enrich uranium or work in this direction. And such monitoring is indeed being carried out. If the laser enrichment method proves its simplicity and efficiency, work on weapons-grade uranium can begin where it is not really needed. Therefore, while the laser method is somehow undermined.

The molecular method, based on the fact that at infrared or ultraviolet frequencies, the 235 UF 6 gas selectively absorbs the infrared spectrum, which later makes it possible to use the method of dissociation of excited molecules or chemical separation, can also be attributed to laser methods.
The relative content of U-235 can be increased by an order of magnitude already in the first stage. Thus, one pass is sufficient to ensure sufficient uranium enrichment for nuclear reactors.

Explanations for the "molecular" method with chemical separation.
Benefits of laser enrichment:
- Electricity consumption: 20 times less than for diffusion.
- Cascade: the number of cascades (from 0.7% to 3-5% for U-235) is less than 100, compared to 150,000 centrifuges.
- The cost of the plant is significantly less.
- Environmentally friendly: less hazardous uranium metal is used instead of uranium hexafluoride.
- The need for natural uranium is 30% less.
- 30% less tailings (dump storage).
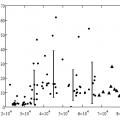
Comparison of indicators of different methods

Uranium enrichment in Russia
Currently, there are four processing plants in Russia:
That's all. Thank you for your attention.
In the message of the Iraqi ambassador to the UN Mohammed Ali al-Hakim dated July 9, it says that at the disposal of the extremists ISIS (Islamic State of Iraq and the Levant). The IAEA (International Atomic Energy Agency) hastened to declare that the previously used nuclear substances by Iraq have low toxic properties, and therefore materials seized by the Islamists.
A source in the US government familiar with the situation told Reuters that the uranium stolen by the militants is most likely not enriched, so it can hardly be used to make nuclear weapons. The Iraqi authorities have officially notified the United Nations of this incident and called on "to prevent the threat of its use," RIA Novosti reports.
Uranium compounds are extremely dangerous. About what exactly, as well as about who and how can produce nuclear fuel, tells AiF.ru.
What is Uranium?
Uranium is a chemical element with atomic number 92, a silvery-white glossy metal, in the periodic table of Mendeleev is designated by the symbol U. In its pure form, it is slightly softer than steel, malleable, flexible, is contained in the earth's crust (lithosphere) and in seawater, and in its pure form is practically does not occur. Nuclear fuel is made from uranium isotopes.
Uranium is a heavy, silvery-white, shiny metal. Photo: Commons.wikimedia.org / Original uploader was Zxctypo at en.wikipedia.
Uranium radioactivity
In 1938 the German physicists Otto Hahn and Fritz Strassmann irradiated the uranium nucleus with neutrons and made a discovery: capturing a free neutron, the uranium isotope nucleus fissions and releases enormous energy due to the kinetic energy of fragments and radiation. In the years 1939-1940 Julius Khariton and Yakov Zeldovich for the first time, they theoretically explained that with a small enrichment of natural uranium with uranium-235, it is possible to create conditions for the continuous fission of atomic nuclei, that is, to give the process a chain character.
What is enriched uranium?
Enriched uranium is uranium that is obtained using technological process of increasing the fraction of 235U isotope in uranium. As a result, natural uranium is separated into enriched uranium and depleted uranium. After the extraction of 235U and 234U from natural uranium, the remaining material (uranium-238) is called "depleted uranium" because it is depleted in the 235th isotope. According to some reports, about 560,000 tons of depleted uranium hexafluoride (UF6) are stored in the United States. Depleted uranium is two times less radioactive than natural uranium, mainly due to the removal of 234U from it. Due to the fact that the main use of uranium is energy production, depleted uranium is a product of little use with low economic value.
In nuclear power, only enriched uranium is used. The uranium isotope 235U has the greatest application, in which a self-sustaining nuclear chain reaction is possible. Therefore, this isotope is used as fuel in nuclear reactors and nuclear weapons. The separation of the U235 isotope from natural uranium is a complex technology that few countries can implement. Uranium enrichment makes it possible to produce atomic nuclear weapons - single-phase or single-stage explosive devices, in which the main energy output comes from the nuclear fission reaction of heavy nuclei with the formation of lighter elements.
Uranium-233, artificially obtained in reactors from thorium (thorium-232 captures a neutron and turns into thorium-233, which decays into protactinium-233 and then into uranium-233), may in the future become a widespread nuclear fuel for nuclear power plants (already now there are reactors that use this nuclide as fuel, for example KAMINI in India) and the production of atomic bombs (critical mass about 16 kg).

The core of a 30 mm caliber projectile (GAU-8 cannon of the A-10 aircraft) with a diameter of about 20 mm from depleted uranium. Photo: Commons.wikimedia.org / Original uploader was Nrcprm2026 at en.wikipedia
Which countries produce enriched uranium?
- France
- Germany
- Holland
- England
- Japan
- Russia
- China
- Pakistan
- Brazil

10 countries providing 94% of world uranium production. Photo: Commons.wikimedia.org / KarteUrangewinnung
Why are uranium compounds dangerous?
Uranium and its compounds are toxic. Aerosols of uranium and its compounds are especially dangerous. For aerosols of water-soluble uranium compounds, the maximum permissible concentration (MPC) in the air is 0.015 mg / m³, for insoluble forms of uranium, the maximum permissible concentration (MPC) is 0.075 mg / m³. When it enters the body, uranium acts on all organs, being a general cellular poison. Uranium is practically irreversible, like many other heavy metals, binds to proteins, primarily to the sulfide groups of amino acids, disrupting their function. The molecular mechanism of action of uranium is associated with its ability to suppress enzyme activity. First of all, the kidneys are affected (protein and sugar appear in the urine, oliguria). With chronic intoxication, disorders of the hematopoiesis and nervous system are possible.
Uranium for peaceful purposes
- A small amount of uranium gives the glass a beautiful yellow-green color.
- Sodium uranium is used as a yellow pigment in painting.
- Uranium compounds were used as paints for painting on porcelain and for ceramic glazes and enamels (they are painted in colors: yellow, brown, green and black, depending on the oxidation state).
- At the beginning of the 20th century, uranyl nitrate was widely used for enhancing negatives and coloring (toning) positives (photographic prints) in brown.
- Alloys of iron and depleted uranium (uranium-238) are used as powerful magnetostrictive materials.
Isotope - varieties of atoms of a chemical element that have the same atomic (ordinal) number, but different mass numbers.
An element of the III group of the periodic table, belonging to the actinides; heavy, weakly radioactive metal. Thorium has a number of applications in which it sometimes plays an irreplaceable role. The position of this metal in the periodic table of elements and the structure of the nucleus predetermined its use in the field of peaceful uses of atomic energy.
*** Oliguria (from the Greek oligos - small and ouron - urine) - a decrease in the amount of urine excreted by the kidneys.
The article describes why uranium is enriched, what it is, where it is mined, its applications and what the enrichment process consists of.
The beginning of the atomic era
Such a substance as uranium has been known to people since ancient times. But unlike our time, they used it only to create a special glaze for ceramics and some types of paint. Natural uranium oxide was used for this, deposits of which can be found in various quantities on almost all continents of the world.
Much later, chemists also became interested in this element. So, in 1789, the German scientist Martin Klaproth managed to obtain uranium oxide, which in its parameters was similar to metal, but it was not. It was only in 1840 that the French chemist Peligo synthesized real uranium - heavy, silvery, and which Dmitry Mendeleev introduced into his table of periodic elements. So why do you need to enrich uranium and how does it happen?
Nowadays

In fact, natural is not much different from the rest. These are massive rusty cobblestones, which are mined in the most common way - they blast the layers of deposits and transport them to the surface for further processing. The fact is that this natural substance contains only 0.72% of the isotope U235. This is not enough for use in reactors or weapons, and then after sorting it is converted into a gaseous state and uranium enrichment begins.
In general, there are many methods for this process, but the most promising and used in Russia is gas centrifugation.
A gaseous uranium compound is pumped into special installations, after which they are spun up to tremendous speeds, and heavier molecules are separated from light ones and grouped at the walls of the drum.
Then these fractions are separated and one of them is converted into uranium dioxide - a dense and solid substance, which is then packed into a kind of "tablets" and fired in an oven. It is for this that uranium must be enriched, since the percentage of the U235 isotope at the outlet is an order of magnitude higher, and it can be used both in reactors and in weapons systems.
Export

If we give a simplified example, then the enrichment of this element is essentially similar to the production of iron - in its original, natural form, these are worthless pieces of ore, which are then converted into strong steel by various processing.
Also in the press you can often hear the fact that many less developed countries in comparison with the same Russia often ask themselves the question of how to make enriched uranium?
The fact is that this process, given the example of gas centrifugation, is very complex, and not everyone can build such installations. Moreover, not one single piece is needed, but a whole cascade of them. In order to understand their technical level, it is worth saying that these "drums" rotate at a speed of 1500 rpm and without stopping. Record - 30 years! Therefore, some countries buy enriched uranium from Russia.
Where is uranium mined in Russia?
Mining of 93% of uranium ore is carried out in Transbaikalia, near the city of Krasnokamensk. Enriched uranium in Russia is produced by JSC TVEL.
Application

The process of converting to a high performance compound is sorted out, but why is it needed? Let's take a look at the two most basic directions.
First, of course, they provide electricity to entire cities, power autonomous spacecraft to explore the far corners of our solar system, stand on submarines, icebreakers, research ships.
Second, it is true that it is worth clarifying - it is uranium in bombs that has not been used for a long time, it has come to replace it. It is obtained by means of special irradiation in low-enriched uranium reactors.
Often, even in the years of the USSR, there was an opinion that especially dangerous criminals or "enemies of the people" were exiled to uranium mines so that they would atone for their guilt with their fleeting labor. And naturally, they didn't stay there for a long time because of the radiation.
In fact, this is not the case. There is no particular danger in working in such a mine, natural ore is little radioactive, and a person, if placed in a mine without a hitch, will die rather from lack of sun and fresh air than from radiation sickness.
Nevertheless, the working conditions of the workers are sparing, only 5 hours a day, and many have been working there for generations, debunking the myth about the terrible destructiveness of such production.
And by the way, they make the cores of weapons shells. The fact is that uranium is much heavier and stronger than lead, as a result of which such damaging elements are more effective, and they also tend to ignite as a result of destruction, after mechanical action on them.
So we figured out why enriched uranium is needed, where it is used and for what purpose.
ENRICHMENT OF NUCLEAR FUEL, separation of the well-fissionable isotope of uranium, uranium 235, from the predominant isotope, uranium 238. Gaseous uranium (VI) fluoride undergoes diffusion separation, which uses a series of baffles with ... ... Scientific and technical encyclopedic dictionary
ENRICHMENT- (1) blowing the introduction of oxygen into the atmospheric air to intensify the technological process during metal smelting (see), (2) O. of minerals, a combination of various methods of processing ferrous, non-ferrous and noble metal ores, coal, etc. ... ... Big Polytechnic Encyclopedia
Uranium ore processing A set of processes for the primary processing of mineral uranium-containing raw materials, aimed at separating uranium from other minerals that make up the ore. In this case, there is no change in the composition of minerals, but only their ... ... Nuclear power terms
uranium ore beneficiation- A set of processes for the primary processing of mineral uranium-containing raw materials, aimed at separating uranium from other minerals that make up the ore. In this case, there is no change in the composition of minerals, but only their mechanical separation from ... ... Technical translator's guide
Radiometric enrichment of ore ore processing processes based on the interaction of various types of radiation with matter. In the technology of radiometric concentration of ores, two types of processes are distinguished: Radiometric sorting ... ... Wikipedia
- (English magnetic separation, magnetic concentration of minerals; German magnetische Aufbereitung f der Bodenschätze) mineral processing based on the action of an inhomogeneous magnetic field on mineral particles from ... ... Wikipedia
- (a. chemical refining; n. chemische Aufbereitung; f. concentration par voie chimique, enrichissement chimique; and. tratamiento quimico, preparacion quimica, elaboracion quimica) technology of primary processing of ores, collective and ... ... Geological encyclopedia
Uranium is the main energy source of nuclear power, generating about 20% of the world's electricity. The uranium industry covers all stages of uranium production, including exploration, development and ore processing. Recycling ... ... Collier's Encyclopedia
Almost ready to go ... Wikipedia
Fuel cell of a nuclear reactor Nuclear fuel is a substance that is used in nuclear reactors to carry out a nuclear fission chain reaction. Contents 1 General information 2 Classification ... Wikipedia
Books
- The Rose of Isfahan, Michel Gaven, 2000s. Iran. An earthquake with great destruction and casualties occurs in the area of the city of Isfahan. Realizing that they cannot cope on their own, the Iranian authorities are forced to apply for ... Category: